One of the significant disadvantages of nickel-cadmium (ni-cad) batteries is that after shallow discharge cycles, the unused portions of the electrodes “remember” the previous cycles and cannot sustain the required discharge beyond the depth of the previous cycles. The capacity is lost and can only be restored by slowly discharging and charging the batteries completely through costly and time-consuming maintenance periods that require the batteries to be pulled from service (generally, outside the application). VRLA DRY CELL AGM or GEL batteries do not exhibit this “use it” or “lose it” capacity-robbing effect known as memory.
It is important to note, however, that if any lead-acid battery is left unattended and discharged for extended periods of time, its ability to do its job will be diminished and eventually extinguished.
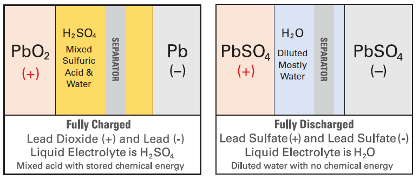
Discover VRLA DRY CELL AGM and GEL batteries are designed using proven gas recombination technology which removes the need for regular water addition by controlling the evolution of hydrogen and oxygen during charging. This means that the oxygen typically produced on the positive plates of all lead-acid batteries is absorbed by the negative plate through a porous medium (see Figure 1) without being vented.
The porous medium in an AGM Dry Cell battery is the woven fibreglass mat. The porous medium in a GEL Dry Cell battery is the cracks in the GEL electrolyte. This suppresses the production of hydrogen at the negative plate. Water (H20) is produced instead, retaining the moisture within the battery. It never needs watering and should never be opened as this would “poison” the battery with additional oxygen from the air.
The retained oxygen produces an overpressure within the cell. This is normal. The battery’s high-quality sealing valves will not open at too low pressure because this would allow too much oxygen to escape and be irretrievably lost. If the highly controlled and defined opening pressure is achieved, the sealing valves will open quickly to release overpressure caused by the accumulated gas. Under normal operating conditions, this gas consists mainly of hydrogen. Under unfavourable conditions (high charge voltages at high temperatures, for instance), oxygen would also escape.
In quality VRLA DRY CELL AGM and GEL batteries, the quantity of electrolyte as a ratio to active material (plates and oxides) is controlled in such a way as to allow the battery to attain an expected performance and design life under normal operating conditions, taking into account:
- The rate of recombination
- The corrosion of the positive electrode (oxygen consumption)
- The losses by diffusion through the cell container
What is an AGM Battery?
An AGM battery, short for Absorbent Glass Mat battery, is a rechargeable battery that utilizes a fibreglass mat to absorb and contain the electrolyte, typically sulfuric acid. During the charging process, water molecules within the battery break down into hydrogen and oxygen gasses. The gasses are trapped by the porous fibreglass mat and subsequently recombined with the water. AGM batteries may also incorporate a one-way, pressure relief valve system to facilitate recombination. Because AGM batteries only contain enough electrolytes to keep the mat wet, they are light, spill-resistant, and safe.
What is a Flooded Battery?
A flooded battery, sometimes called a wet battery, is a traditional type of lead-acid battery that uses a liquid electrolyte, typically sulfuric acid. The electrolyte completely covers the lead positive and negative plates within the battery cells. Unlike AGM batteries, flooded batteries are not sealed and do not have special pressurized sealing vents. During the charging process, gasses produced at the positive plate are released through the vent plugs. As a result, flooded batteries require regular maintenance including adding distilled water to compensate for water loss during the charging process.
AGM vs Flooded Batteries
In this video case study, the choice between AGM, flooded, and lithium batteries for a material handling (ride-on order picker) application are discussed by Discover Battery’s VP of Business and Product Development Jimmy Au and Delta-Q Technologies’ Customer Success Manager Hiroshi Hasegawa:
When choosing between AGM and Flooded batteries, there are several important differences to be aware of.
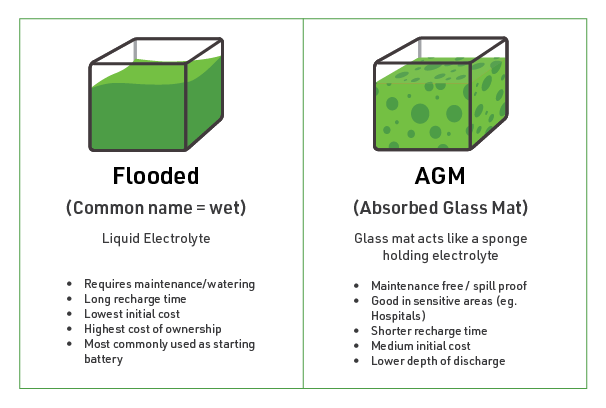
Sealed vs Open Design: AGM batteries offer several benefits over flooded batteries due to their sealed design. First, AGM batteries are virtually maintenance-free. The electrolyte is absorbed into a fibreglass mat, eliminating the need for regular water additions. In contrast, flooded batteries require regular maintenance, including adding distilled water to compensate for water loss during the charging process, cleaning terminals, and ensuring proper ventilation.
Additionally, their sealed design allows AGM batteries to be installed in various orientations, offering flexibility and versatility in applications. Flooded batteries, on the other hand, must be installed upright. Because of their open design and the excess electrolytes they contain, they may spill if tipped or punctured, potentially causing damage or corrosion.
It is important to note that some brands of flooded or wet automotive batteries are also marketed and sold as sealed or maintenance-free. However, they are still flooded cell batteries. They do not have the same pressurized venting system as AGM batteries and will spill electrolytes if not kept upright.
Safety: Flooded batteries are not air transportable without special containers and they typically can’t be shipped via express courier or parcel post due to the risk of electrolyte spills. In contrast, AGM batteries have no DOT restrictions.
There are additional safety concerns when it comes to using flooded batteries around sensitive electronic equipment. The off-gassing can cause corrosion within the electrical circuits or other damage.
Cost: Although flooded batteries are typically less expensive upfront, they may end up costing more over the battery’s lifespan. This is due to the cost of regular maintenance including labour and service products, such as distilled water. Additionally, accidental spills and off-gassing during charging and discharging can lead to additional expenses and environmental concerns.
Performance: Flooded batteries are susceptible to acid stratification, which occurs when the heavier acid in the electrolyte separates from the water. This can cause premature battery degradation or failure. Acid stratification is accelerated by factors such as frequently operating in a partial state of charge, seldom receiving a full charge, being left unused for long periods, or even exposure to extreme temperatures. Acid stratification can also be caused by the battery being regularly micro-cycled between 3%-17.5% depth of discharge, high-cycled between 17.5% - 30% depth of discharge, or deep-cycled beyond 50% depth of discharge. This is especially true for the automotive starting type batteries and dual-purpose marine/RV combination cycling/starting batteries that many manufacturers are selling as low-cost alternatives to true deep cycle batteries.
AGM batteries are not affected by acid stratification to the same extent as flooded batteries. The fibreglass mat completely absorbs and constrains the acid which makes it more difficult for the acid to diffuse out of the water and accumulate at the bottom of the battery’s cells.
Figure 1 compares wet flooded vented battery gassing and VRLA DRY CELL AGM and GEL battery recombination.
Figure 1 - Vented Battery Gassing and VRLA DRY CELL Battery Recombination.
AGM and flooded batteries offer several distinct advantages and disadvantages. When selecting a battery, factors such as the specific application, desired lifespan, maintenance requirements, and budget should all be considered. For options that require frequent deep-cycle discharges, low maintenance, and improved performance, AGM batteries are often the preferred choice. However, for less demanding applications, and budget-conscious consumers, flooded batteries may still be a viable option.
GEL and AGM batteries are Valve-regulated lead-acid (VRLA) recombinant technology batteries. Both GEL and AGM batteries are considered to be of a starved electrolyte (DRY CELL) design. Both are sealed and considered non-hazardous - nonspillable.
In an AGM or GEL battery, the electrolyte does not flow like a normal liquid.
In AGM batteries, all liquid electrolyte is trapped in a sponge-like mat of woven glass fibre separator material.
In a GEL battery, the electrolyte is mixed with a silicate additive which immobilizes (changes) the electrolyte into a GEL-like material consistency.
The “acid-starved” condition of GEL and AGM batteries protects the plates during heavy deep- discharges. The more acid-starved the batteries are, the more protection is given to the active materials. The Sulfuric acid in the battery electrolyte is fundamental to the discharge process. When the acid is depleted, the discharge process cannot go on.
Due to the physical properties of the GEL electrolyte and the batteries' inherently higher internal resistance, GEL battery power declines faster than an AGM battery’s as the temperature drops below 32°F (0°C).
AGM batteries excel for high current, high power applications and in extremely cold environments. GEL batteries tend to live longer in hot climates.
AGM batteries are better replacements for FLOODED batteries than GEL batteries when used in alternator-equipped applications. GEL batteries require lower (precisely controlled) charging voltages (mainly lower finishing voltages) than AGM or Flooded types. AGM battery charge voltages are more similar to Flooded charge voltages (14.4V – 14.7V) than GEL charge voltages (13.5V to 13.8V).
At room temperature, the difference between GEL and AGM batteries for higher current, high power applications is minimal.
High Deep Cycle demands on battery-powered equipment, the increased cyclic demand and parasitic electrical loads brought about by the use of start-stop technologies, increased electrical systems in modern vehicles, and the frequent partial state of charge (PSOC) operation combine to accelerate the #1 cause of declining battery life expectancy: Acid Stratification.
Acid stratification happens naturally in flooded lead-acid batteries. The fluid in a battery is called the electrolyte. The electrolyte is a mixture of sulfuric acid and water. Acid is heavier than water and is fundamental to the electrochemical charge and discharge process in a lead-acid battery. Acid stratification happens when the heavier acid in the battery’s electrolyte separates from the water and assembles at the bottom of the battery’s cell, creating an area of very high specific gravity electrolyte.
What are the Effects of Acid Stratification?
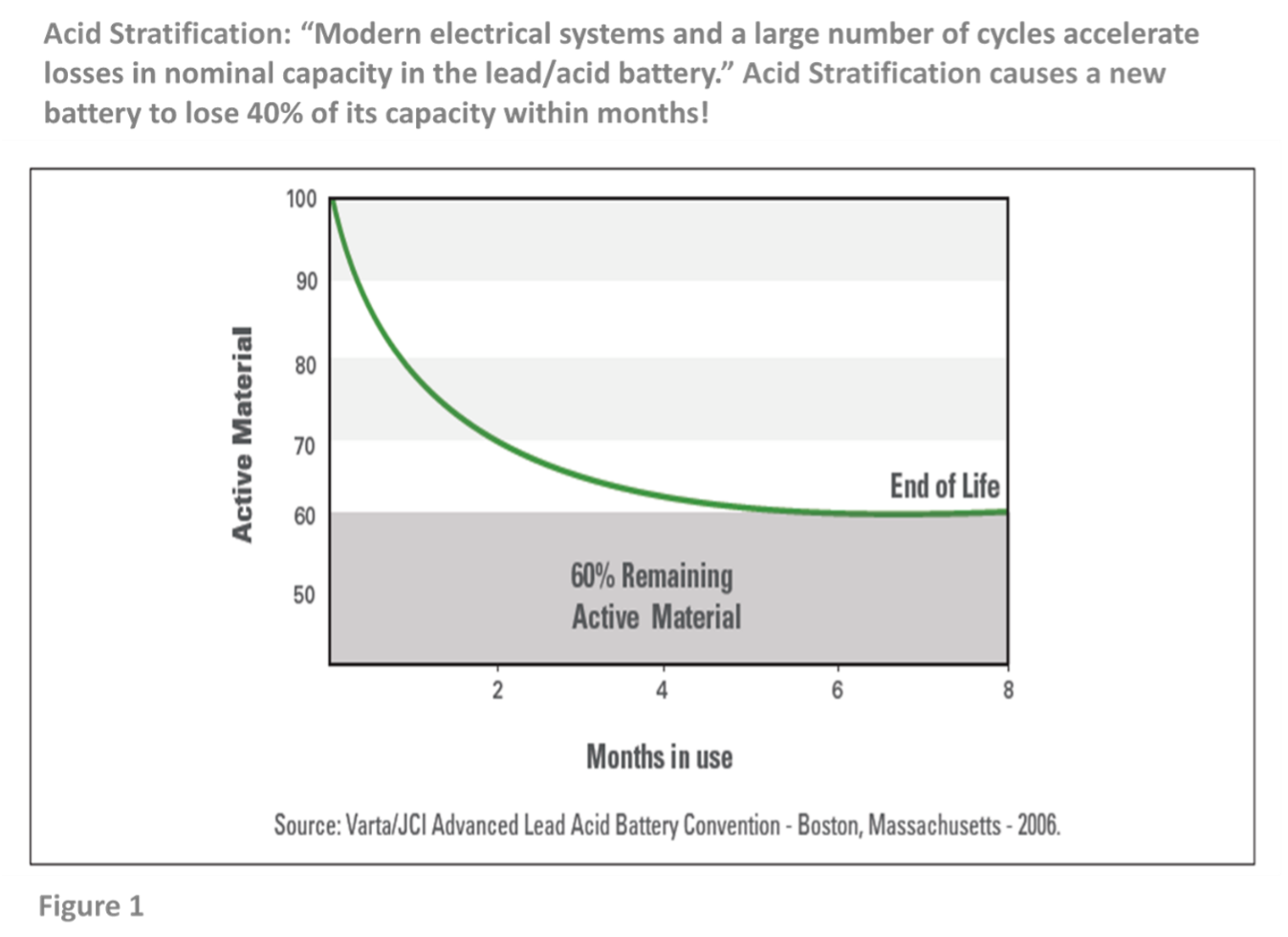
- In wet flooded batteries, acid stratifies (sinks to the bottom of the battery’s cells). The upper portion of the battery’s plates is left subject to low specific gravity electrolyte (now mostly water). The upper portion of the plate is rendered inactive and is no longer capable of supporting discharge capacity. Under these conditions, the useful active material in the battery will be reduced by as much as 40% within six months of normal use, creating “dead lead” or “inactive active material.” (See Figure 1)
- The stratified acid at the bottom of the battery’s cell focuses discharge activity to the bottom of the cell, causing the bottom part of the plate to work overtime. While the bottom part of the plate gets excessively discharged, the top part of the plate receives most of the charging activity. As a result, acid stratification can cause a battery’s dynamic charge acceptance1 (“DCA”) to decline by 50% to 70% within six months of installation, increasing alternator wear and tear and decreasing fuel efficiency.
- Since electrical current moves more easily through water (top part of the cell) than it does through acid (bottom part of the cell), stratified acid concentrates charging current and charging heat at the upper part of the plate, accelerating corrosion which dramatically lowers the battery’s cranking power (“CCA”).
- Stratified acid promotes increased internal resistance, lower conductivity and accelerated sulfation on the lower part of the plates, reducing the battery’s dynamic charge acceptance. This means a sulphated battery will only accept a surface charge, resulting in a false positive state of charge readings to vehicle computers and battery testers. So, a battery may appear fully charged but only provide low “CCA” and “AH”/”RC.”
Acid stratification is accelerated if:
- the battery operates in a partial state of charge (PSOC)
- the battery seldom receives a full charge
- the battery is constantly micro-cycled between 3% - 17.5% DOD as in start-stop vehicles
- the battery is regularly high cycled between 17.5% and 30%
- the battery is regularly deep-cycled beyond 50% DOD
- the battery is used or exposed to extreme temperatures,
- and the battery is left standing for long periods.
All of these conditions contribute to premature battery failure.
