How does a battery recharge?
The process is the same for all lead-acid batteries: FLOODED, GELL and AGM.
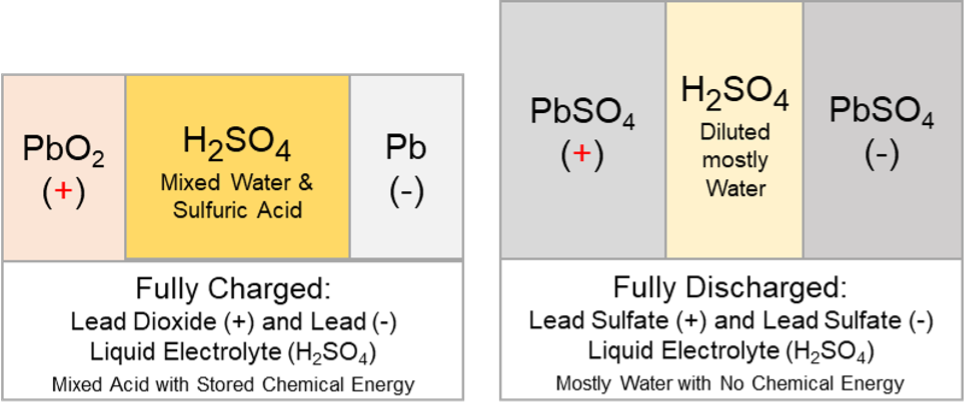
The actions that take place during discharge are the reverse of those that occur during the charge.
The discharged material on both plates is lead sulphate (PbSO4). When a charging voltage is applied, charge flow occurs. Electrons move in the metal parts, and ions and water molecules move in the electrolyte.
Chemical reactions occur at both the positive and negative plates, converting the discharged material into charged material. The material on the positive plates is converted back to lead dioxide (PbO2), and the material on the negative plates is converted back to lead (Pb) during the charge.
Sulfuric acid is produced at both plates, and water is consumed at the positive plate. If the voltage is too high, other reactions will also occur; Oxygen is ripped from water molecules at the positive plates and released as a gas; Hydrogen gas is released at the negative plates – unless oxygen gas can reach the negative plates first and “recombine” into H20. A battery will “gas” near the end of the charge because the charge rate is too high for the battery to accept.
A temperature-compensating voltage-regulating charger, which automatically reduces the charge rate as the battery approaches the fully charged state, eliminates most of this gassing. It is essential not to charge batteries for long periods at rates which cause them to gas because they use water, which in sealed valve-regulated batteries cannot be replaced. Of course, no battery should be overcharged for an extended period, even at low rates using so-called “trickle charges.”
In a fully charged battery, most sulphate is in sulfuric acid. As the battery discharges, some of the sulphates begin to form on the plates as lead sulphate (PbSO4). As this happens, the acid becomes more diluted, and its specific gravity drops as water replaces more sulfuric acid. A fully discharged battery has more sulphates in the plates than in the electrolyte. A battery left in this discharged state or continually undercharged will prematurely fail. This failure condition is often referred to as sulfation.